作者简介:王倩文 (1994—),女,硕士在读,研究方向: 环境细胞生物学。全宰植(JEON Jae-sik, 1987—),男,博士,研究方向: 环境细胞生物学。
#全宰植同为第一作者
目的 本研究旨在研究浓度较低PM10(10 ng/L)引起的细胞活性与细胞毒性的变化。评估了在比韩国环境部发布的可吸入颗粒物PM10环境标准(100 μg/m3)更低浓度的PM10对人肺上皮细胞 A549 活性和乳酸脱氢酶(LDH)释放的影响。方法 使用用于水溶性四唑盐(WST-8)和乳酸脱氢酶 (LDH) 释放测定的商业试剂盒,没有PM10的 RPMI 用作阴性对照,细胞活力通过阴性对照释放的活细胞线粒体中的琥珀酸脱氢酶与WST-8的结合进行表达。相对于未经处理的对照细胞和不含 FBS的培养基的基础 LDH 释放来表达细胞毒性。分别评估暴露于 PM10溶液(10 ng/L和 10 μg/L) 0、4、12 h后的人肺泡上皮细胞(A549)的细胞活性和细胞毒性。结果 在低于韩国环境部发布的可吸入颗粒物PM10环境标准浓度的PM10浓度下,发现 PM10对 A549 细胞活性具有时间依赖性影响,细胞释放的 LDH 测试中,没有观察到显著差异。结论 PM10在较低浓度下对 A549 细胞活性产生时间依赖性影响,其对A549细胞的细胞毒释放影响需要多种浓度和多种时间数据来证实。而低浓度PM10对肺癌发病率的影响有待进一步研究。研究结果证明低 PM10 浓度会显着影响肺泡上皮细胞的活力,从而为更好地了解 PM10对人体呼吸系统健康的潜在有害影响提供参考材料。
Objective In this study, we aimed to investigate the changes in cell proliferation and cytotoxicity caused by a lower concentration of PM10(10 ng/L) than the PM10 environmental standard (100 μg/m3) issued by the Korean Ministry of Environment, human lung epithelial cell (A549) proliferation capacity and lactate dehydrogenase (LDH) release were evaluated at PM concentrations close to the domestic and foreign environmental standards for inhalable particulates.Methods Commercial kits for the determination of tetrazolium base (WST-8) binding and LDH release were used. RPMI medium without PM10 was used as a negative control. In the WST-8 assay, cell viability was expressed relative to the binding of succinate dehydrogenase to WST-8 in the mitochondria of living cells in the negative control. Cytotoxicity was expressed relative to the basal LDH release by untreated control cells and the medium without FBS. Cell proliferation and cytotoxicity of human alveolar epithelial cells (A549) were examined to evaluate PM10exposure (10 ng/L and 10 μg/L) for 0, 4, 12 h.Results At a lower concentration of PM10than the PM10 environmental standard issued by the Korean Ministry of Environment, PM10 had a time-dependent effect on A549 cell proliferation. No significant difference was observed in LDH released by the cells.Conclusions PM10 exhibits time-dependent effects on cellular activity at low concentrations. However, its toxicity to A549 cells should be studied at other concentrations and over longer study periods to confirm its effects. The effect of low-concentration PM10 on the incidence of lung cancer merits further investigation. Our findings demonstrate that low PM10 concentration can significantly affect the viability of alveolar epithelial cells. The findings could help better understand the potential negative effects of PM10 on the overall respiratory health of humans worldwide.
Particulate matter (PM) is a widespread air pollutant that comprises a complex mixture of solid and liquid particles suspended in the air. It is directly formed from natural or artificial sources, as well as from chemical reactions between previously emitted particles in the atmosphere[1]. Particles with an aerodynamic diameter (hereinafter, referred to as diameter) of ≤ 10 µ m are commonly referred to as PM10, whereas those with a diameter of ≤ 2.5 µ m are fine PM2.5. These particles can remain in the atmosphere for an extended duration and enter the body through breathing, accumulate in the trachea or lungs, and ultimately affect overall physical health[2].
PM is a complex mixture of elemental carbon, sulfate, nitrate, ammonium, metals, and many other organic compounds[3], which can adsorb organic pollutants and heavy metals, thereby substantially increasing the risk of carcinogenesis, teratogenesis, and mutations. In particular, the risk of death associated with heart and lung diseases has increased by 6% and that by lung cancer has increased by 8%[4]. Dust particles that remain in the atmosphere from several hours to several days increase the incidence of respiratory diseases, including asthma, bronchitis, and pneumonia. Furthermore, they cause cardiovascular diseases, stroke, conjunctivitis, skin irritation, meningococcal meninges inflammation, and Valley fever[5]. A recent study on the effect of dust particles on living cells revealed that an increase in dust concentration inhibits cell proliferation[6, 7, 8]. Therefore, in the present study, we aimed to explore the effects of soluble substances in haze on toxicity and cell viability.
A standard sample of ambient dissolved fine dust (PM10-LIKE; certificate of analysis: ERM-CZ120) from the Joint Research Centre Institute for Reference Materials and Measurements (Geel, Belgium) was used to evaluate toxicity.
A model for human lung alveolar carcinoma epithelial cells (A549) was purchased from the Korean Cell Line Bank (Seoul, Republic of Korea) and cultured in Dutch Modified (1X) RPMI 1640 medium (LM 011-07) from Welgene (Gyeongsan-si, Republic of Korea) under a humidified atmosphere (5% CO2) at 37 ℃. All media were supplemented with 10% fetal bovine serum (FBS) from Corning (Glendale, USA), 100 units/mL penicillin, and 100 units/mL streptomycin. The cells (1 × 104 cells /well) were seeded in 96-well plates, incubated overnight at 37 ℃ under a 5% CO2 atmosphere for 24 h.
PM10 was diluted in the Dulbecco's phosphate-buffered saline (DPBS) solution from Welgene (Gyeongsan-si, Republic of Korea). The cells were treated with PM10 at a final concentration of 10 ng/L and 10 μ g/L for 0, 4, or 12 h.
The effect of PM on cell viability was determined using the Quanti-MAX WST-8 (tetrazolium salt) Cell Viability Assay Kit (Biomax, Seoul, Republic of Korea). Briefly, 10 μ L of WST was added to each well and incubated for 0.5 h. Absorbance was measured at 450 nm using a plate reader. RPMI without fine dust was used as a negative control and changes in cell viability were determined in triplicate.
LDH release from the cells to the culture medium was monitored using the Quanti-LDH PLUS Cytotoxicity Assay Kit (Biomax, Seoul, Republic of Korea). The cytotoxicity of PM10 was measured by determining LDH concentration using a colorimetric assay. Aliquots (100 μ L) of the cell culture medium were collected from each well and placed in new microtiter plates. LDH (100 μ L) was added to each well and the plates were incubated for 0.5 h, and then the absorbance of the sample was measured at 490 nm using the Flex Station 3 Multi-Mode Microplate Reader (Molecular Devices, USA). Each experiment was performed in quadruplicate. Cytotoxicity was expressed relative to the basal LDH release by untreated control cells and the medium without FBS. The test was replicated three times, and the amount of LDH released by the PM-exposed cells was expressed as a percentage of that released by the non-exposed cells.
Statistical analyses were performed using one-way analysis of variance with Microsoft Excel 2019 (Build 14430.20306), and the results are expressed as the mean±standard error of the mean. Statistical significance was set at P< 0.05.
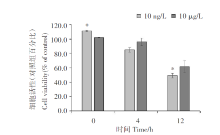
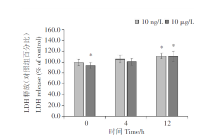
The PM10 extract (10 ng/L and 10 μ g/L) was found to inhibited A549 cell proliferation in a time-dependent manner (Figure 1). In addition, the chemical parameters did not inhibit cell proliferation. Next, extracellular LDH enzyme activity was determined to further assess the number of membrane-damaged cells. After 12 hours the LDH level released from A549 cells was observed significantly increasing in both of PM10 exposed groups compared with that from control cells (Figure 2).
In this study, we found that PM10 significantly inhibits the proliferation of alveolar epithelial cells in a time- and concentration-dependent manner. These findings are consistent with those of previous studies, which demonstrated that cell proliferation significantly decreases in a concentration-dependent manner in PM2.5-treated A549 cells[9, 10, 11]. Nonetheless, the two PM10 concentrations tested (10 ng/L and 10 μ g/L) did not exert significant cytotoxic effects[12], which contrasted with the result of previous studies, which showed a significant increase in LDH release in a concentration-dependent manner in PM2.5-treated A549 cells[10, 11]. These different effects of PM on cells may be explained by the low concentrations used in the present study. As shown in Figure 1, the Pvalue of the cell viability data under 10 ng/L PM10 treatment for 0 and 12 h with that of non-PM10-exposed cells at the indicated time points was low. Therefore, it can be considered that there is significant difference in cell viability at 10 ng/L. We used the 10 µ g/L treatment to compare with higher concentrations, but no obvious difference in cell activity at higher concentration was found. The results in this study indicate that the effects of PM10 depended on time, and its relationship with concentration was not significant. In this study, 10 ng/L was the experimental group, and higher concentration of 10 µ g/L was set as an experimental comparison.
Numerous studies have experimentally used relatively high concentrations of PM10; however, the PM10 concentration is low in the atmosphere, and the amount of PM10 that can enter the alveoli is very small compared to the concentration dependence[10, 11]. Only a few studies have performed experiments with such low concentrations (10 ng/L), which are lower than the standard PM10 concentrations in domestic and foreign haze environments (100 μ g/L). Indeed, cell morphology and release of LDH are unaffected after 24 h of exposure to 125 and 250 μ g/L PM[13].
Oxidative stress is often considered to be a primary effect of PM toxicity. PM-induced reactive oxygen species (ROS) formation has been widely reported and is associated with metals and organic species within particles[14]. In addition to the well-known role of some metals in the generation of ROS, the implications of organic compounds were recently confirmed[15]. However, further experiments are necessary to confirm these findings.
Measurement of cell proliferation and viability has become a core investigational approach in the field of life sciences[16]. The demand for sensitive, reliable, rapid, and simple methods has led to the development of various detection methods, including the tetrazolium salt (WST)-based assay. Notably, unlike 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), which splits into water-insoluble formazan crystals and must be dissolved after cleavage, WST produces water-soluble cleavage products: 2, 3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) and 3-(4, 5-dimethylthiazol-20yl)-(3-carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H-tetrazolium (MTS), that can be measured without an additional solubilization steps. Furthermore, WST is more stable than XTT and MTS[17].
Herein, A549 cells were used as the experimental model in the present study as a means to intuitively mimic the potential effects of PM10 on lung health. Furthermore, they have been used by the European Commission to address and explore PM components and their subsequent effects. In addition, the release of LDH was analyzed as a means to evaluate the effect of PM on cell integrity and viability, since the loss of intracellular LDH and its release into the culture medium are indicators of cell membrane damage leading to irreversible cell death[18]. Reliability, speed, and simple evaluation are some of the advantages of this test[19], which has been used as an indicator of cadmium chloride cytotoxicity in liver (HepG2) cells[20].
Despite the efforts to ensure the accuracy and sensitivity of the tests performed, this study still has certain limitations. First, the PM10 formulation (mixed with DPBS) and the concentrations used may differ from the PM commonly present in the atmosphere. Moreover, there are differences between the PM10 data retrieved from the European Commission website and the results obtained for actual PM in the atmosphere. The PM10 developed in the laboratory is synthetic, and its shape is relatively full and round, whereas the edges and corners of the PM10 shape under natural conditions are more likely to break the cell membrane and affect cell ability. To overcome these limitations, additional comparative experiments should be performed in the future.
PM is a complex mixture of elemental carbon, sulfate, nitrate, ammonium, metals, and several other organic compounds[21]. The viability of A549 cells significantly increases (12.3%) after metal removal from PM, indicating the important contributions of metal components to PM toxicity[22, 23]. Exposure to PM10 particles increases the production of tumor necrosis factor (TNF)-α in human monocytes and promotes apoptosis in A549 cells[24]. This indicates that exposure to air contaminants induces an inflammatory state, modulates the immune system, and increases the expression of molecules that favor respiratory infections, thereby affecting the respiratory system[13]. Therefore, based on these results, we will study the effects of heavy metals on the cellular activity, the release of LDH, and the inflammatory factors IL-6, IL-8, and TNF in human non-epithelial cells in the future. We will also further determine the relationship between air pollution and viruses responsible for major lung infections in recent years.
In this study, PM10 at a low concentration (10 ng/L), close to the haze environment limit (150 μ g/m³ ), was considered. It was found that PM10 affected the proliferation ability of A549 cells in a time-dependent manner at this low concentration. Therefore, the toxicity of PM10 in A549 cells should be studied at various concentrations and times. The effect of low PM10 concentrations on the incidence of lung cancer requires further investigation. This study provides additional evidence that low PM concentrations can considerably affect the viability of alveolar epithelial cells, thereby improving our understanding of the potentially negative effects of PM on the overall respiratory health of humans worldwide.
Ethics approval and consent to participate This study was approved by the Institutional Review Board Committee of the Dankook University (No. 2019-12-007) and was conducted in accordance with the Declaration of Helsinki.
Competing interests The authors declare no competing interests.
编辑:黄艳
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|